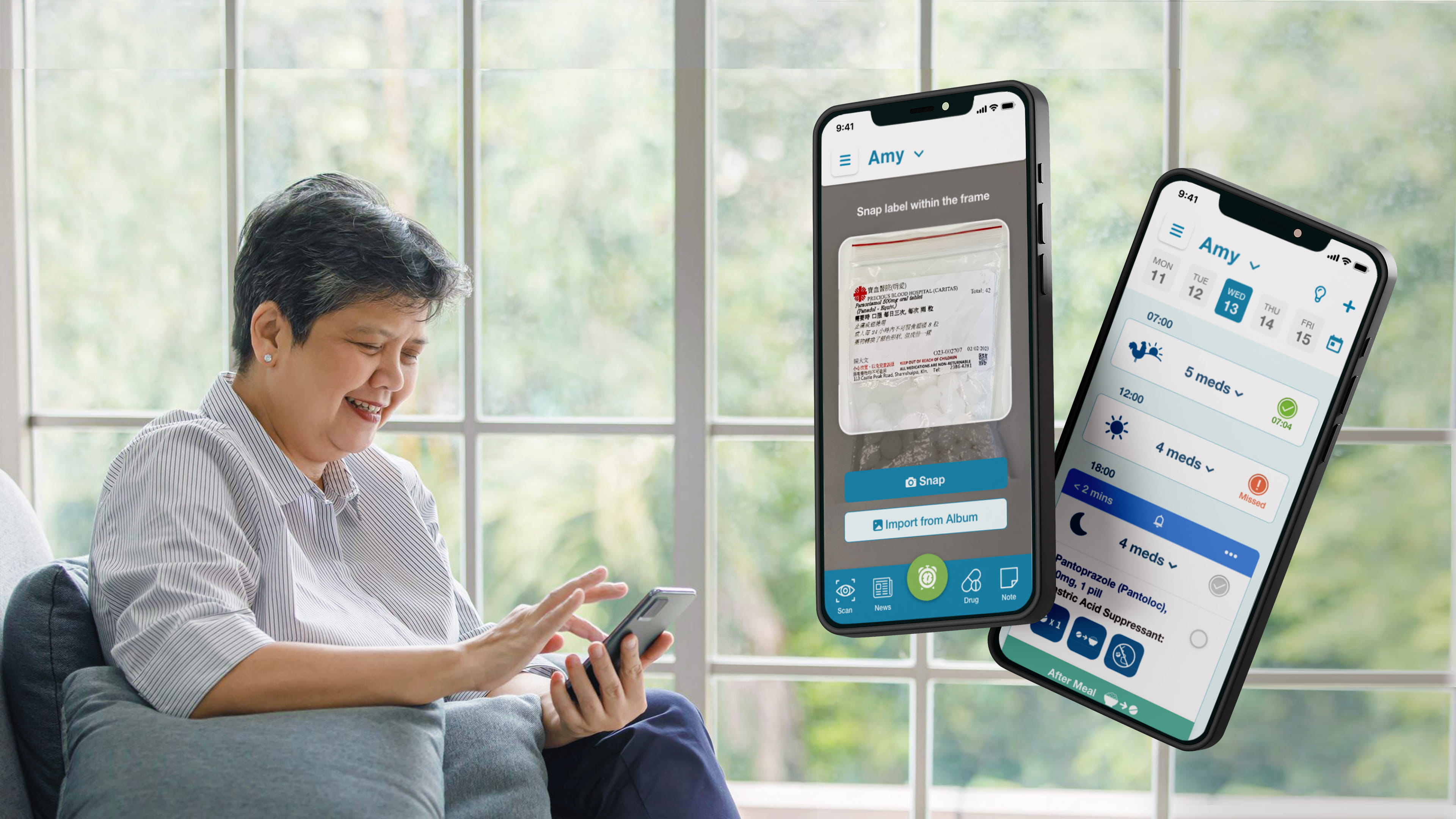
Labeling machines assist in printing UDI labels for medical system equipment

UDI (Unique Device Identification) labels are a marking system used for medical devices. Every medical item intended for shipment to the United States or some European countries must be marked with a UDI label, aimed at improving the safety and efficacy of medical devices. UDI labels are typically displayed in the form of barcodes or QR codes, making them easy to scan and track in medical environments. The main goal of introducing the UDI system is to enhance the traceability of devices and promote medical safety.
The current medical device market is increasingly complex. With advancements in technology and the proliferation of device types, ensuring the safety and effectiveness of medical devices has become crucial. The emergence of the UDI label system addresses these challenges. UDI labels are created by manufacturers based on the device identifier (DI), expiration date, lot number, and serial number.
Four Key Benefits of UDI Labels
- Improved Traceability: The UDI system allows each medical device to be tracked throughout its lifecycle, which is crucial for device recalls and investigations of adverse events.
- Enhanced Safety: Accurate identification and tracking enable healthcare organizations to better manage devices, reducing the risk of errors and misuse.
- Strengthened Data Collection: UDI can help healthcare organizations collect and analyze data regarding device usage, thereby improving clinical practices and enhancing patient safety.
- Simplified Regulatory Compliance: For manufacturers, the UDI system aids in meeting regulatory requirements and streamlining the reporting and product registration process.
The UDI label system represents a significant advancement aimed at enhancing the safety and efficacy of medical devices and strengthening the regulation and management of medical products. As the system is promoted and applied, the healthcare industry will become safer and more efficient. But which products require UDI labels?
Who Needs UDI Labels?
- Medical Device Manufacturers
- Large Manufacturers: Produce various medical devices, such as pacemakers, artificial joints, imaging devices, etc.
- Small and Startup Companies: Develop new medical devices, such as eyewear or medical aesthetic skincare products. Regardless of size, they must comply with UDI standards.
- Medical Device Wholesalers and Distributors: These companies are responsible for transporting medical devices from manufacturers to healthcare institutions and must be able to identify and track the devices.
- Hospitals and Clinics: When using and managing medical devices, they need to track the usage and history of each device.
- Government and Regulatory Agencies: Such as the FDA (U.S. Food and Drug Administration) and European medical device regulatory bodies, which are responsible for establishing and enforcing UDI regulations.
- Logistics and Supply Chain Management Companies: These companies need to effectively track and manage the transportation and storage of medical devices.
- Medical Research Institutions: When conducting clinical trials or research, accurate identification and tracking of the involved medical devices are necessary.
- Health Insurance Companies: In claims processing and data analysis, UDI labels are needed to ensure the accuracy and compliance of devices.
All companies involved in the production, distribution, use, and regulation of medical devices must adhere to UDI standards to enhance the safety and efficacy of devices and strengthen their tracking and management.
UDI Label Content and Production Process
The first step is to determine the label content. Personnel need to collect all necessary device data, including device name, model, manufacturer information, production batch, serial number, and expiration date. Then, identifiers need to be selected (determine the Device Identifier (DI) and Production Identifier (PI) and allocate them according to relevant regulations).
The design, format, and layout of the label must also adhere to standards, including the format of the barcode or QR code, fonts, colors, and other visual elements, ensuring compliance with relevant UDI standards and regulations set by regulatory agencies.
Once the label design is completed, specialized software is used to encode the device information into a barcode or QR code. This step ensures that the data can be scanned and recognized. Then, suitable printing techniques (such as thermal transfer, laser printing, etc.) are used to print the designed labels on label materials. The materials can be paper, plastic, or other durable materials to suit different usage environments.
Brother professional label maker combined with the free software P-Touch Editor, allows you to save time and quickly obtain detailed asset information, generating UDI labels to track asset information. Businesses can extract text, barcodes, and other printing content from Excel data files without manual data entry.
The production process of UDI labels involves multiple steps, from information collection to label design, generation, and application. Each step must strictly follow standards and regulations to ensure the safety and effectiveness of the final product. This not only helps to enhance the traceability of medical devices but also strengthens the trust of patients and healthcare institutions in the devices.
Medical device manufacturers are responsible for the entire design and generation process of the UDI label, including determining label content, designing format, generating barcodes or QR codes, and printing labels. However, some companies may seek professional label suppliers to provide label printing and data encoding services. If a company needs to print in-house, they may consider using a Brother professional label maker, easily installed with P-Touch Editor on a computer, allowing the creation of custom templates and database connections. Once data is linked to objects, UDI labels can be easily produced.
(For detailed production processes, you may consult Brother professionals.)

- Fast print speed of up to 60mm per second
- Support WiFi and Ethernet
- Advanced cutter with easy-peel function
- Optional Touch Panel with backlit LCD display to print custom labels without the use of a PC
- Industrial label design software for PC and Mac
- Up to 360 x 720dpi print resolution

- Fast print speed of up to 60mm per second
- USB & Wireless connectivity
- Advanced cutter with easy-peel function
- Optional Touch Panel with backlit LCD display to print custom labels without the use of a PC
- Industrial label design software for PC and Mac
- Up to 360 x 720dpi print resolution

- Bluetooth connection via the Design&Print2 app
- Choose from 3.5, 6, 9, 12, 18, 24, 36mm label width
- PC Connectable and using Ptouch Editor software for label editing
- Built-in Li-ion battery
- Fast print speed of up to 20mm per second
- Auto Cutter and half cutter